Tom Scherzer, Gabriele Mirschel and Katja Heymann
Leibniz Institute of Surface Modification (IOM), Permoserstr. 15, D-04318 Leipzig, Germany. E-mail: [email protected]
Introduction
Polymer coatings play an important role in our daily life. They are widely used in numerous applications, which range from decorative and protective coatings to coatings with specific functional properties. Polymer coatings can be made by various technologies such as drying of solvent- or water-based lacquer formulations, reaction of two-component systems, or thermal curing of resin mixtures. A particularly efficient and versatile method to produce polymer coatings is UV curing of multifunctional monomers and oligomers such as acrylates, methacrylates, epoxies and vinyl ethers. This method is characterised by high production speeds and superior environmental sustainability as well as producing a wide range of potential properties and the high quality of the resulting coatings.
The functional properties of UV-cured coatings strongly depend on several process parameters. The two most important variables are the conversion (degree of cure) of the functional groups and the thickness of the applied layer. For instance, scratch and abrasion resistance depend on the hardness of the layer, which is directly related to the degree of cure. Coatings for exterior applications have to show a high resistance against weathering, which can be only achieved if the conversion is largely completed. Insufficient conversion also increases the soluble fraction, which can be extracted from the coating and is one of the most critical parameters for packaging materials (e.g. for sensitive goods such as foods, pharmaceuticals etc.). A sufficient conversion is also indispensable for further processing of the coating, e.g. with respect to wipe resistance. Furthermore, a number of functional and other application properties (e.g. barrier behaviour, mechanical resistance, hiding power etc.) strongly depend on the thickness of the coating.
On the other hand, the extent of the conversion depends on numerous key variables such as the intensity of the incident UV light, the line speed, the composition and the homogeneity of the reactive formulation, the ambient conditions during irradiation (temperature, inertisation, humidity etc.) and other factors. Whereas some of them can be easily controlled, even under the conditions of technical UV curing, this is hardly possible for effects like ageing, pollution or failure of UV lamps, differences between various batches of the lacquer formulation, or other unexpected influences. The thickness of the applied coating may fluctuate as well due to variations of viscosity, temperature, line speed etc.
In order to ensure a constantly high quality of the manufactured products, the compliance of the actual level of the conversion and the thickness of the coating with the requirements determined by the specific application has to be controlled continuously. In the past, near infrared (NIR) spectroscopy has been proven to be a powerful technique for the monitoring of various polymerisation processes. However, the typical thickness of UV-cured coatings is in the range of a few micrometers only and hence much lower than the sample thickness used in most other applications of NIR spectroscopy. In combination with the low absorptivities of bands in the NIR, the low thickness might be expected to make the analysis of such samples under process conditions a difficult task. However, it will be shown here that NIR reflection spectroscopy is able to follow even small variations of both the conversion and the thickness of thin coatings and that it accordingly can be used as an efficient in-line measuring method.
Instrumentation
A dedicated process analyser system was developed according to the specific requirements of in-line measurements on thin UV-cured coatings. It is based on a photodiode array spectrometer (Kusta 4004; LLA, Berlin, Germany) and a tailor-made probe head, which is linked to the spectrometer by an optical fibre. The TE-cooled InGaAs detector with 256 pixels (Sensors Unlimited, Inc.) was set to cover a spectral range from 1470 nm to 1950 nm. The tungsten halogen lamp used as light source is integrated into the gold-coated reflection probe head. In order to prevent post-polymerisation of the coatings by the short-wavelength part of the lamp emission, a UV filter is mounted in front of the probe head. Spectra of transparent film samples are measured in transflection mode, i.e. they are taken against a ceramic retroreflector, which is placed behind the foil.
When thin transparent foils of optically high-grade polymers (e.g. polypropylene, polyamide, polyester) are used as a substrate, the thickness of which is in the range of or only a little higher than the wavelength of the probe light (i.e. foils up to ~ 20 µm), interference fringes appear that completely mask the spectrum and prevent any analysis if a clear coating is applied to the foil. Such interferences can be efficiently suppressed by a specific diffuser plate, which is mounted in front of the probe head, and by a tilt of the probe head against the sample since the amplitude of the fringes depends on the angle of incidence of the probe light.1
For in-line monitoring, the probe head was installed above the moving web of a roll coating machine or the conveyor belt of a pilot line for the irradiation of coatings on panels and plates.
Calibration
The preparation of a set of well-defined calibration and validation samples as well as the calibration process is generally a laborious and time-consuming matter. However, in respect of the investigation of photo-polymerised coatings it is even more complex. The specific preparation of UV-cured coatings with a predetermined conversion is mostly difficult to achieve, and the careful characterisation of cross-linked polymers is usually a labour-intensive task. This will be outlined here for a typical calibration procedure. Layers of an acrylic clear coat formulation were applied to thin polypropylene foil with a nominal thickness of 10 µm and irradiated in a UV curing unit. In order to achieve samples with a wide range of conversions, samples with different photoinitiator concentration were prepared and irradiated with different UV doses (i.e. at different UV intensities and with different line speeds). The actual conversion after UV exposure was determined with Fourier transform infrared (FT-IR) transmission spectroscopy. FT-IR spectra were taken at several points on each sample, whereas NIR spectra were recorded continuously while the sample beam focus was scanned across the sample. In both cases, the spectra were averaged before further processing.
The data set from 160 samples was split into a calibration set (60%) and a validation set (40%), and a chemometric model was built up using the Partial Least Squares (PLS) algorithm and the test set validation method. Normalisation was the only pretreatment that was applied to the data. The resulting calibration curve is shown in Figure 1. The performance of the developed model was tested with an additional set of independent samples, which were included neither in the calibration nor in the validation set. Their conversion was predicted with the model and compared with FT-IR reference data. A very close correlation was found, which clearly proves the high predictive power of the created chemometric model.
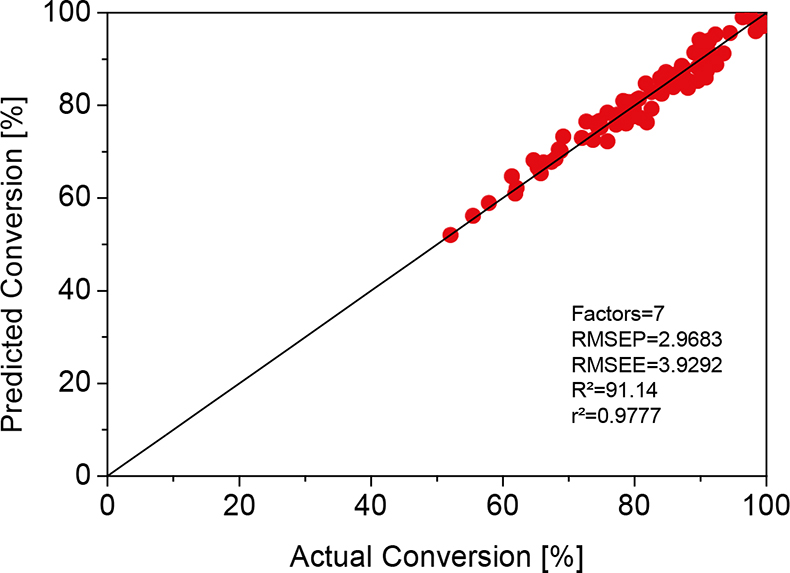
Figure 1. PLS calibration of the NIR reflection spectra of UV-cured acrylic clear coats to the conversion determined by FT-IR transmission spectroscopy.
In spite of the potential of multivariate calibration routines, it is obvious that the effort that is necessary for the calibration step might impede the use of chemometric procedures in coating and curing technology, in particular since coating applications are changing frequently within small companies. Naturally, a new calibration is required for each job. For this reason, a less sophisticated alternative method was developed, which allows a less time-consuming quantitative analysis of the NIR spectra. It is based on the first overtone of the C–H stretching vibration of the vinyl bond which appears at 1620 nm in the NIR spectrum of acrylates and methacrylates and at about 1612 nm for vinyl ethers. Since the band is well separated from most of the other C–H absorptions between 1680 nm and 1800 nm, the conversion of UV-cured coatings can be determined directly from the ratio of the integrals of the band before and after irradiation of the sample. The accuracy of this method was confirmed in a previous investigation by comparing the conversion data obtained in this way with results from FT-IR spectroscopy.2
Monitoring of the thickness of coatings is always based on chemometric methods, since calibration samples with well-defined thicknesses can be easily prepared, e.g. by use of a set of Baker applicators. The actual thickness after UV irradiation can be determined with a thickness gauge or by determination of the coating weight.
In-line monitoring
The predictive power of the PLS model was demonstrated in a real technical coating process. The acrylate formulation was applied to polypropylene foil with a coating weight of 15 g m–2 corresponding to a thickness of 10 µm. The NIR probe head was mounted above the web of a pilot-scale roll coating machine (see Figure 2), and NIR spectra were recorded continuously at a rate of 140 spectra per minute.

Figure 2. Installation of the probe head on a pilot-scale roll coating machine at IOM.
A record of a typical test trial is shown in Figure 3. In technical UV curing processes, the most important factor that significantly affects the conversion is the irradiation dose that is applied to the coating. Unintended dose changes may result from various causes such as ageing, pollution or failure of the UV lamps. In order to simulate changes of the dose, both the irradiance and the web speed were varied alternately. The irradiance is given as percentage of the maximum output of the UV lamp. It is apparent that the acrylate conversion increases or decreases according to the applied irradiation dose. After each change of the dose an almost instantaneous response of the conversion can be observed. The line speed was stepwise increased to 120 m min–1 at the end of this trial. However, in further test runs the conversion was followed also at even higher operating speeds of the coating machine. The time resolution of the method appears to be sufficient for process control, i.e., it allows for immediate intervention if a significant deviation from the target value is detected.
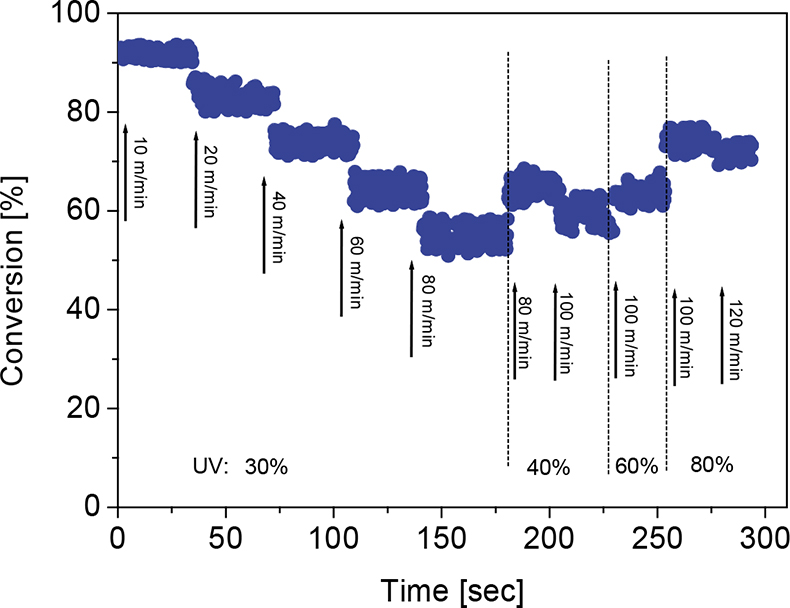
Figure 3. In-line monitoring of the acrylate conversion of a clear coat after irradiation with various UV doses.
Besides acrylic clear coats, the conversion after UV (or electron beam) exposure has been also monitored in pigmented systems,2 radiation-curable adhesives,2 epoxy/vinyl ether formulations3 and in coatings based on nanocomposite materials.4
The thickness of UV-cured coatings was followed by NIR-based in-line monitoring as well. It can be determined with a precision of less than 1 µm at best.5 If both conversion and thickness of the layer are to be controlled, this can be achieved either by two probe heads or by application of a PLS2 algorithm. Calibration to more than one parameter requires much higher effort for the calibration procedure; however, the precision of the prediction of both parameters is at least comparable or sometimes even better than those of PLS1-based single measurements. The PLS2 approach is also useful to correct the conversion for incidental changes of the thickness of the coating, e.g. after changes of the web speed.
The method developed for UV-cured polymer coatings was also adapted for monitoring of the thickness of thin silica layers made by VUV-curing of perhydropolysilazane.6 Whereas the typical thickness of UV coatings is in the range of 5–50 µm (and is already rather low with respect to measurements by NIR spectroscopy), the thickness of SiOx barrier layers is even lower and lies in the sub-micron range, i.e., in the range of 100–700 nm. Since the NIR spectra of inorganic layers do not show overtone and combination absorption bands, analysis was based on tiny differences in reflectance. In spite of these conditions, which are quite exceptional for NIR spectroscopy, it could be shown that in this case the method in fact is able to predict the thickness of the silica layers with an error in the order of 20%.
Conclusions
NIR reflection spectroscopy was shown to be a powerful method for in-line monitoring of important process parameters in polymer coating technology such as the conversion after UV curing and the thickness of the coatings. Quantitative analysis was predominantly based on multivariate calibration models. Alternatively, univariate procedures could be developed in order to reduce the effort required for calibration. The time resolution of the method and the precision of the results were found to be sufficient for the use of this technique for process and quality control.
Acknowledgements
The authors wish to acknowledge the financial support by the AiF association (grant numbers KF 0336101KFK1 and KF 0189603FK6). Moreover, we thank Udo Trimper (IOM) for technical assistance and LLA GmbH (Berlin, Germany) for cooperation.
References
- T. Scherzer, R. Mehnert and H. Lucht, Macromol. Symp. 205, 151 (2004). https://doi.org/10.1002/masy.200450114
- T. Scherzer, S. Müller, R. Mehnert, A. Volland and H. Lucht, Polymer 46, 7072 (2005). https://doi.org/10.1016/j.polymer.2005.05.142
- T. Scherzer and M.R. Buchmeiser, Macromol. Chem. Phys. 208, 946 (2007). https://doi.org/10.1002/macp.200600649
- T. Scherzer, S. Müller, R. Mehnert, A. Volland and H. Lucht, JCT CoatingsTech 3, 30 (2006).
- T. Scherzer, K. Heymann, G. Mirschel and M.R. Buchmeiser, J. Near Infrared Spectrosc. 16, 165 (2008). https://doi.org/10.1255/jnirs.800
- T. Scherzer, G. Mirschel, K. Heymann, L. Prager and M.R. Buchmeiser, Appl. Spectrosc., in press.

