Kevin M. Smith
Molecular Spectroscopy Facility, Space Science and Technology Department, CCLRC Rutherford Appleton Laboratory, Chilton, Didcot, Oxfordshire, OX11 0QX, United Kingdom
The second part of this article appeared in Spectroscopy Europe 19/2
Introduction
The origins of the Molecular Spectroscopy Facility (MSF) at the Rutherford Appleton Laboratory (RAL) can be traced back to the 1980s when a Facility was set up to make infrared absorption measurements in support of studies on the Jovian atmosphere. It was during this work that unique facilities were built that make the MSF such a useful research tool today. The centrepiece of the MSF is the long path absorption cell capable of providing optical paths of up to one kilometre at temperatures as low as 200 K. The requirement for long path measurements at low temperatures is not unique to planetary studies and by the early 1990s the bulk of the Facility’s work was related to the understanding of Earth’s atmosphere. Since then the United Kingdom (UK) Natural Environment Research Council (NERC) have provided funding for UK academic researchers to visit the MSF to collect valuable data in support of grant-funded projects and studentships. Currently, NERC-funded work accounts for 85 % of MSF business and up to 15 different projects are supported by the Facility each year. As well as providing access to state-of-the-art equipment and high-quality spectroscopic data, the MSF is a valuable source of training for students.
Traditionally, the end use of the data recorded at the MSF has been in the closely allied fields of Earth observation and atmospheric science. For example, a large amount of data has been produced on the absorption of mid-infrared radiation by industrial compounds, such as hydrofluorocarbon species. These data are used by climate modellers to investigate possible impacts on the Earth’s radiation balance, who in turn can provide advice to policymakers and regulators. Over the years the Facility has developed to meet the needs of a diverse range of research topics within the NERC scientific remit, ranging from the study of leaves and bones to jet engine emissions and water dimers. Table 1 gives a brief outline of some of the research carried out at the MSF in recent years and the science it has enabled.
Table 1. Recent research carried out at the MSF.
Capability | Recent examples of NERC research the MSF has enabled |
High spectral resolution | Improving climate models: |
Broadband and continuous spectral coverage | Aerosol studies: |
Flexible measurement configurations | Characterisation of greenhouse gases: |
Variable optical path length | Molecular cluster ions: |
Controlled sample conditions | Quantitative water dimer measurements: |
Chemistry and hazardous substances | Studies of peroxyacetyl nitrate (PAN): |
Instrumentation
The MSF is based around a high-resolution Bruker IFS 125HR Fourier transform spectrometer (FTS), that provides continuous coverage of the electromagnetic spectrum from the terahertz to the ultraviolet. The instrument is based on a Michelson interferometer with a maximum optical path difference of six metres, providing a resolving power of greater than 106 in the mid-infrared. This instrument is supplemented by a number of fibre-optic interfaced miniature grating-based spectrometers, a medium-resolution FTS and a mid-IR quantum cascade laser system. The unique feature of the Facility is the wide variety of sample cells and accessories that may be used in combination with these spectrometers. As appropriate, transmission, reflection or emission measurements may be made on gases, vapours, aerosols, bulk liquids, solid surfaces and powders, and custom-made systems have been designed to produce, purify and handle a wide variety of substances safely and efficiently. Always samples are held within controlled environments; temperature, pressure, humidity, flow rate and even ion number density can be controlled, continuously monitored and recorded.
Recent NERC-funded projects
To give a flavour of the type of work undertaken at the MSF, brief descriptions of four recent research projects are given below.
Water dimers
Water vapour is perhaps the most important radiatively-active gas in the Earth’s atmosphere and is therefore highly relevant to climate studies. Water vapour has an extremely complex spectrum made up of tens of thousands of lines associated with rotational and rotational–vibrational transitions, ranging from the microwave to the visible and ultraviolet parts of the spectrum. Underlying the spectral lines are broad continuum absorptions, the precise origins of which have yet to be resolved. Proposed mechanisms for the existence of these continua range from the cumulative effect of absorption in the far wings of lines to absorption by water dimers. Water dimer, (H2O)2, is a complex consisting of two weakly-bonded water molecules.
Currently, many atmospheric radiative transfer models, including those used to model climate change, rely on a semi-empirical model called the Clough, Kneizys and Davies (CKD) continuum.1 The CKD model has been derived from the difference between observed and calculated absorption line spectra, and is extrapolated to spectral regions and conditions that have not been observed. There has been considerable debate about the ability of the CKD continuum to model spectra correctly at the full range of temperature and pressure conditions found in the Earth’s atmosphere. To address this problem the Ma and Tipping (MT) model2 is based on ab initio calculations of far line wings, but this too has so far failed to fully account for the observed water vapour continuum absorptions. Over the years the possibility that water dimer could account for some of the continuum absorption has been debated, and efforts have been made to model the dimer spectrum from ab initio calculations.3 However, the question of whether water dimers can play a significant role in atmospheric absorption remains largely unanswered. This uncertainty is due to two unknowns; first, the strength of the absorption, and second, the abundance of the dimer in the atmosphere. The latter is thought to be dependent on both the partial pressure of water vapour and on the ambient temperature.
Most previous laboratory measurements on the water dimer have used expanded molecular beams or solid matrices at very low temperatures.4–8 A recent literature review found only one previous measurement of what may have been dimer absorption at room temperature.9 These measurements, by Burch, were made some 20 years ago at relatively low spectral resolution. For atmospheric relevance, laboratory measurements should be made under equilibrium and gaseous conditions, and this has been undertaken at the MSF. Using a heated multi-pass gas cell, a series of measurements were made in the infrared part of the spectrum between 2000 cm–1 and 8000 cm–1 in the temperature range 296–340 K. Particular attention was paid to the water vapour bands around 3800 cm–1 and 5300 cm–1. These regions were chosen to provide a balance between dimer absorption intensity, as predicted by theory, and interference from monomer spectral lines. Although the dimer absorptions are expected to be relatively broad and smoothly varying, high spectral resolution was required to resolve the narrow absorption lines of the monomer and separate them from the underlying dimer features.
A range of temperatures and water vapour pressures were studied and the resulting dataset was compared with state-of-the-art spectral simulations. Line-by-line models were used to calculate the contribution from spectral lines and these were subtracted to leave the continuum as a residual absorption. These calculations were based upon the HITRAN10 spectral database, which is an internationally respected compilation of spectral line parameters, and the latest versions of the continuum models. Figure 1 shows a measured and calculated water vapour absorption spectrum in the spectral region 5250–5400 cm–1. This simple comparison clearly shows some wavelength-dependent discrepancies between observations and theory.
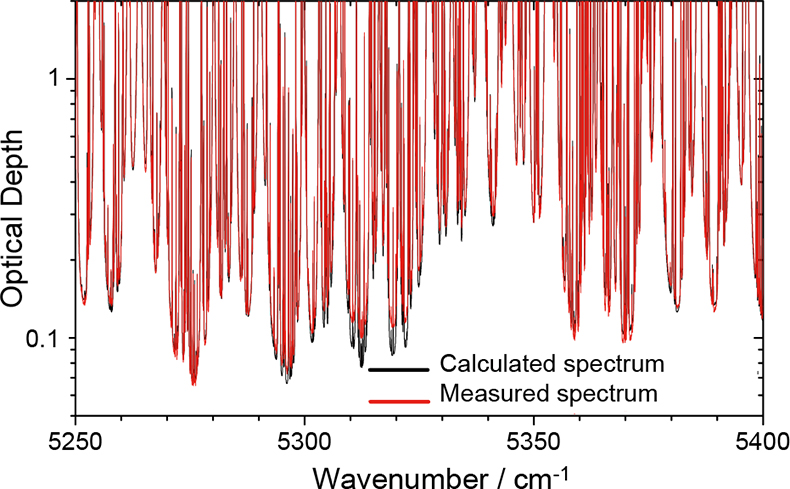
Figure 1. A high-resolution absorption spectrum in the range 5250–5400 cm–1 for 20 hPa of water vapour at 296 K over an optical path of 128 m. The red curve shows a measured spectrum recorded at the MSF, the black curve shows a calculated spectrum based on the HITRAN spectral line database and the latest version of the CKD continuum model. Large discrepancies are apparent between 5290 cm–1 and 5340 cm–1 indicating that a component of the absorption is missing from the calculation in this region.
The red points in Figure 2 represent residual absorption coefficients in the 3400–4000 cm–1 spectral region derived from the difference between and observation and a calculated water vapour line spectrum. The green, pink and blue curves represent three versions of the water vapour continuum, none of which can fully explain the residual absorption features. The cyan curve represents theoretical water dimer absorption. Two assumptions have been made about the dimer; first, that the equilibrium constant (related to the abundance of dimer) at 296 K is 0.038 atm–1, and second, that the half width at half maximum intensity of the absorption features is 31 cm–1. These values provide the best fit to the observations, and since neither are known with any certainty this seems to be a reasonable approach. Also shown are the previous measurements made by Burch.9 Figure 2 indicates that the residual absorption feature is likely to be attributable to a combination of dimer and continuum components. Measurements have been made at the MSF over a range of temperatures and pressures and a major new four-year NERC-funded measurement programme led by the University of Reading will shortly be under way to extend these investigations to shorter and longer wavelengths, targeting both the dimer and the underlying continuum. These data and a full analysis will be the subject of future journal publications.
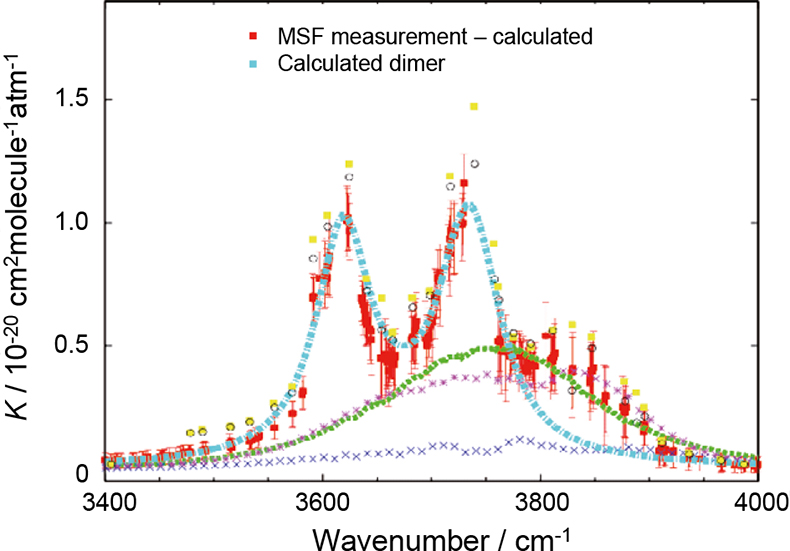
Figure 2. Measured and calculated water vapour continuum absorption coefficients, K, in the 3400–4000 cm–1 spectral region for a temperature of 296 K. The red points show the residual (and uncertainty) between the measured absorption and a calculation based upon HITRAN water vapour line parameters only (i.e. no continuum model was included in the calculation). The cyan curve shows a calculated water dimer spectrum, assuming a half width at half maximum intensity of 31 cm–1 and an equilibrium constant of 0.038 atm–1. The yellow points and open circles show residual features from previous measurements by Burch.9 The green, pink and blue curves show MT-CKD (v1.10),11 CKD (v2.40) and MT versions of the water vapour continuum absorption respectively. This figure is reproduced courtesy of the University of Reading.
Aerosols
An aerosol is defined as a suspension of small particles in a gas. The particles may be liquid or solid and in the atmosphere their sources are both natural and anthropogenic. Aerosols may come directly from a source, e.g. dust or sea spray blown from the surface, or indirectly, e.g. from chemical reactions. Aerosols are thought to play a significant role in the radiation balance of Earth’s atmosphere, through direct and indirect means. The direct effect is attributed to the absorption and scattering of solar radiation by the particles themselves. The indirect means are numerous and complex but mainly result from effects on cloud formation, lifetime and albedo. Aerosols also play a role in atmospheric chemistry, providing surfaces for reactions between other species to occur. It is important that the magnitude and sign of these effects are represented accurately in climate and chemical models, however, there are currently large uncertainties. More information about the role of aerosols in the atmosphere and climate has been published by the Intergovernmental Panel on Climate Change (http://www.grida.no/climate/ipcc_tar/wg1/index.htm).12 Therefore information about the composition and size of particles in the atmosphere and their distributions is urgently required. The refractive indices of aerosols must be measured under carefully controlled laboratory conditions to satisfy two requirements: first, to provide radiative properties for a range of compositions and size distributions for direct input into climate models, and secondly to provide reference data to enable the remote sensing of aerosol composition and distribution from satellite instruments.
The earliest measurements on aerosols at the MSF were made during the mid to late 1990s13,14 when an investigation was underway into polar stratospheric cloud (PSC) aerosols. PSCs play a role in the depletion of stratospheric ozone, and their effect on stratospheric chemistry depends on the chemical composition and physical properties of the particles. PSCs can exist in various physical forms; as liquid, crystalline or amorphous particles. This work is on-going and has produced some of the first mimic-PSC extinction spectra at relevant atmospheric conditions. Previously spectra had been recorded elsewhere from thin-film samples,15 with limited applicability to atmospheric aerosol. For instance, super-cooled liquid droplets can be produced in the aerosol cell, whereas substrate-induced freezing limits the temperature range of thin-film liquids. Since those early days much work has been undertaken at the MSF to improve our understanding of the aerosols produced in the laboratory and to control their chemical composition and size distribution. Figure 3 shows mimic PSC aerosol particles flowing through a chemical conditioner during the production process prior to injection into the aerosol cell.

Figure 3. Mimic PSC particles passing through a conditioner. These are used together with bubblers to control chemical composition during the production process before injection into the aerosol cell.
The MSF has built a dedicated 75 dm3 coolable aerosol cell,14 shown in Figure 4, that may be interfaced with any of the Facility’s spectrometers to measure extinction spectra from the far infrared to the ultraviolet. Aerosol is injected at the top of the cell and allowed to descend under gravity past the optical axis of the spectrometer. The temperature, pressure and humidity within the cell can be controlled and monitored to simulate tropospheric or stratospheric conditions.
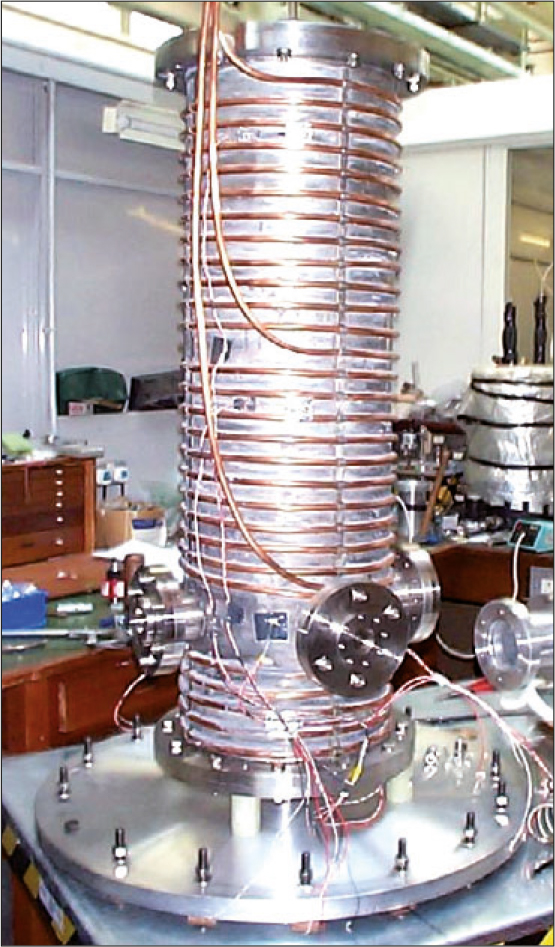
Figure 4. The MSF aerosol cell with the outer jacket removed, showing the internal vessel that contains the sample surrounded by cooling pipes. A vacuum contained within the outer jacket provides thermal insulation. Four viewing ports are positioned towards the bottom of the cell, these may be interfaced with any of the MSF spectrometers. Aerosol is introduced at the top of the cell and is allowed to drift past the viewing ports under gravity. The cell may be cooled to reproduce tropospheric or stratospheric conditions. Nitrogen gas or synthetic air may be flowed into the cell and a vacuum pump used to control the total pressure. Other gases, such as water vapour, may also be injected to study uptake by the aerosol particles and any consequential change in their optical properties.
Methods have been devised, in partnership with Oxford and Open Universities, to produce and characterise many types of aerosols including volcanic and Saharan dust and sea salt aerosol. The particular challenge of mineral aerosols is to produce them in sufficient quantities and with appropriate size distributions. Techniques have been developed to increase the residence time of the aerosol samples during measurements, including the use of ultrasonic traps to overcome rapid fall out of the larger particles due to gravity. Methods of improving measurement sensitivity are currently being developed, including the use of multi-pass optics and quantum cascade laser spectrometers. The latter will be used to probe gas-phase components at high spectral resolution and high sensitivity to measure vapour uptake onto particle surfaces. Figures 5– 7 show example extinction and refractive index spectra for PSC and volcanic ash aerosols.
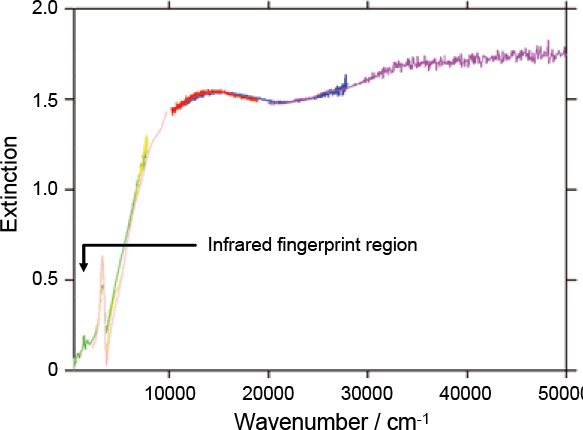
Figure 5. An example mimic PSC extinction spectrum covering the spectral range 300–50,000 cm–1. This spectrum is a composite of data from several spectrometers (as indicated by the differently coloured curves) used simultaneously to span a wide wavelength range from the infrared to the ultraviolet. The finer structure in the infrared part of the spectrum is related to the chemical composition of the aerosol and is effectively a fingerprint. At shorter wavelengths the spectrum is strongly influenced by the physical properties of the aerosol, e.g. the size of the particles.
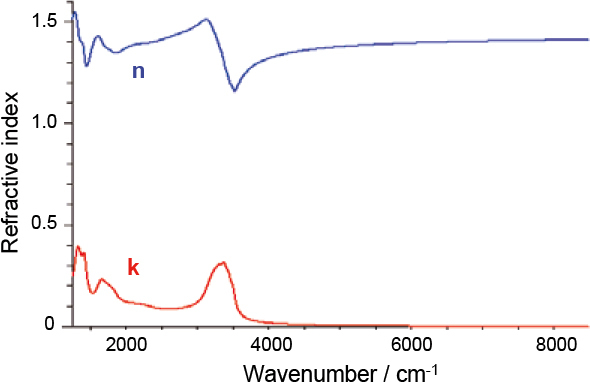
Figure 6. To be useful in climate models or remote sensing applications it is necessary to derive refractive indices from the extinction spectra. The blue and red curves represent the real and imaginary parts of mimic PSC refractive index as a function of wavenumber, corresponding to the scattering and absorption components of extinction, respectively. For supercooled liquid droplets calculating refractive indices is relatively straightforward, since a spherical particle geometry can be assumed.
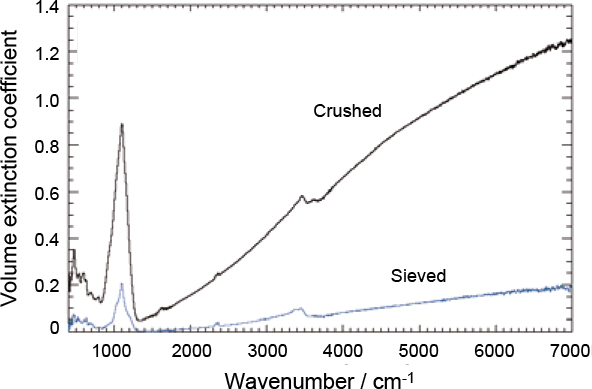
Figure 7. Extinction spectra of volcanic ash recorded at the MSF. The upper and lower curves correspond to crushed and sieved samples, respectively. The difference in the magnitude of the extinction coefficients is simply due to the reduction in the amount of material caused by the sieving process. A spherical particle geometry cannot be assumed for mineral aerosol, therefore it is considerably more challenging to produce accurate refractive indices. Analysis of this data is on-going at the University of Oxford.
to be continued
References
- S.A. Clough, F.X. Kneizys and R.W. Davies, Atmos. Res. 23, 229 (1989). doi: https://doi.org/10.1016/0169-8095(89)90020-3
- Q. Ma and R.H. Tipping, J. Chem. Phys. 116, 4102 (2002). doi: https://doi.org/10.1063/1.1436115
- D.P. Schofield and H.G. Kjaergaard, Phys. Chem. Chem. Phys. 5, 3100 (2003). doi: https://doi.org/10.1039/b304952c
- F. Huisken, M. Kaloudis and A. Kulcke, J. Chem. Phys. 104, 17 (1996). doi: https://doi.org/10.1063/1.470871
- J.B. Paul, C.P. Collier, R.J. Saykally, J.J. Scherer and A. O’Keefe, J. Phys. Chem. A 101, 5211 (1997). doi: https://doi.org/10.1021/jp971216z
- S.A. Nizkorodov, M. Ziemkiewicz and D.J. Nesbitt, J. Chem. Phys. 122, 194316 (2005). doi: https://doi.org/10.1063/1.1899157
- J.P. Perchard, Chem. Phys. 266, 109 (2001). doi: https://doi.org/10.1016/S0301-0104(01)00327-5
- J.P. Perchard, Chem. Phys. 273, 217 (2001). doi: https://doi.org/10.1016/S0301-0104(01)00496-7
- D.E. Burch, Absorption by H2O in Narrow Windows between 3000–4200 cm–1. Tech. Rep. AFGL-TR-85–0036, Hanscom Air Force Base, Massachusetts (1985).
- L.S. Rothman et al., JQSRT 96(2), 139 (2005). doi: https://doi.org/10.1016/j.jqsrt.2004.10.008
- E.J. Mlawer, D.C. Tobin and S.A. Clough, in preparation (MT_CKD code is available at http://rtweb.aer.com/continuum_frame.html).
- Intergovernmental Panel on Climate Change, Climate Change 2001: The Scientific Basis. IPCC Third Assessment Report (2001).
- A. Heathfield, D.A. Newnham, J. Ballard, R. G. Grainger and A. Lambert, Appl. Optics 38, 6408 (1999). doi: https://doi.org/10.1364/AO.38.006408
- R.A. McPheat, S.F. Bass, D.A. Newnham, J. Ballard and J.J. Remedios, J. Geophys. Res. 107, 4371 (2002). doi: https://doi.org/10.1029/2001JD000641
- U. M. Biermann, B.P. Luo and T. Peter, J. Phys. Chem. A 104, 783 (2000). doi: https://doi.org/10.1021/jp992349i

